Gaining intraosseous access: A lifesaving technique for emergencies
Why is intraosseous access effective in acute care and emergency medicine? Why and how should you practice it?
Medical devices play a critical role in helping healthcare professionals offer the best patient care. Both inside and outside of the hospital, personnel apply a variety of devices in their daily work. The number of devices might vary depending on the clinical setup, but the need to keep up to date with usage, software updates, and IT setup persists.
Some medical devices are easy to implement and start using right away. Some are more complex and may require comprehensive training and support across the organization – from end users to paramedics, nurses, physicians, and biomedical technicians. So, before you even get to the buying and implementation of a device, there are some things you can do to lay the groundwork for success. Therefore, we have divided this article into three sections. The first two sections encourage you to consider potential risks related to buying new devices and solutions so you can try and mitigate them. In the third part we dive into the implementation process with our best practices summarized into a 3-step framework.
Hidden costs are costs that are not identified ahead of implementing a new medical device and they might be difficult to identify. Therefore, paying attention to hidden costs sounds like an impossible task. Nevertheless, it is important to consider what comes after having purchased the new medical device, e.g., maintenance, supplies, training, support, and upgrades, which might add extra time and resources to the implementation and usage of the device.
These elements may not have been identified ahead of implementation, and in this regard, we would especially highlight elements related to training as important. Even when medical devices do not include IT interfaces and do not need integrating into existing solutions, training is often a good idea when implementing something new. With training it is ensured that the product is used the intended, most effective way, and users may feel more comfortable using it in their work. Put in another way, it is essential to allocate time and resources to getting to know the medical device and learn about its key features.
Training is fundamental. Still, there are more factors to consider for a successful implementation of new medical equipment. A holistic project management practice including people, processes, and technology need to be considered, not least when implementing connected devices with IT components – which includes a vast amount of today’s medical equipment. Structured management that includes key internal and external stakeholders will help mitigate many risks that may otherwise materialize without thorough planning.
Trying to anticipate hidden costs may be difficult. There are a some more tangible topics that you can plan for in your implementation project. We have listed four prominent ones below.
Patient safety
Without a question, patient safety always comes first. The need to have proper training and follow-up after going live is a must to avoid any mistakes in patient care workflows.
Transition from old to new
Oftentimes, you might have had the existing device for ten years and now that device is getting replaced for an improved one with more innovative features, or something easier to use. Whichever the case, there will be a learning curve, even if training is well executed. If the processes and follow-ups are not managed as part of the implementation, the benefits of the new device may be difficult to realize.
User adoption
Involving people from all levels of the organization is the key to secure smooth implementation and proper usage. There is a need to involve both the management as well as end users and the biomedical technicians. Each user approaches the implementation from their own standpoint and their diverse input helps to build better processes. Failure to involve the right people already in the planning can lower commitment during the training and use phase. In this regard, the innovation-adoption curve can be a helpful tool. It demonstrates that adopting new products takes time, because some users are open to innovations, and some are not. This is a dynamic can be utilized when implementing medical devices, as those who are quick to adopt new products can help get the more apprehensive users onboard.
Costs
New device procurements often have either cost or outcome goals defined. To realize one of the goals, or both, each area of the medical device implementation process – planning, configuration, training, go-live, and support – needs to be managed well. Failure to cover these areas adequately will cost you more money as you grapple with issues such as longer procedures, patient dissatisfaction, and longer discharge time.
We recognize that each implementation project is unique. While the level of complexity may vary, we have found that there are some common denominators to successful medical device implementation.
Overall, it is our belief that implementation done in collaboration with the users is key to a successful outcome. When involving the people affected by the implementation in your healthcare organization, you can approach the implementation more holistically, from several angles, from end user experience to management, IT setup, and security. Moreover, by involving the right people from the start, you establish commitment and trust, which is essential to achieve success.
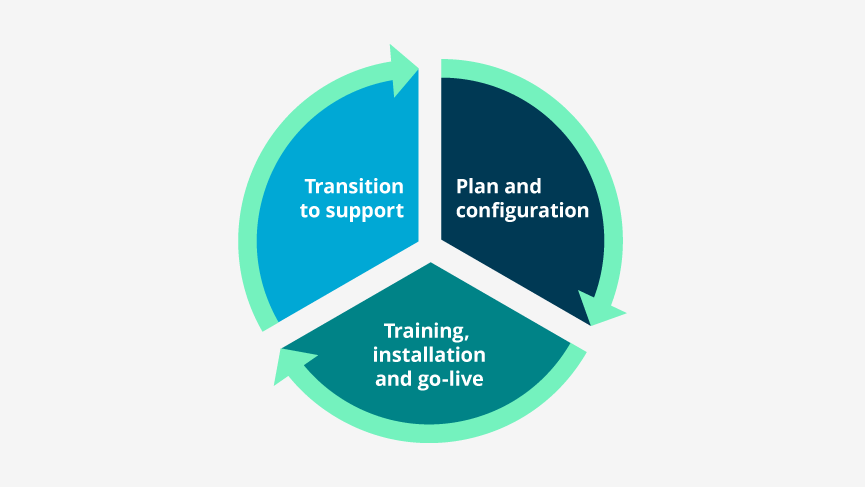
Phase I: Planning and configuration
Together, the supplier and customer form the project team and define the goals in the planning phase. Next, the goals are tied to milestones and measurable metrics. Milestones are important, especially in larger projects, both to manage the project time frame but also to create small wins during the process that keep motivation high.
Next, identify all the stakeholders; users, technical and IT, all in-hospital stakeholders that cooperate or receive data from the medical device. Based on this, you can create a plan for training, configuration, and setup to meet the organization’s specific needs and goals. With clarity and measurable metrics, you can focus on the right activities and tasks to match your desired outcome.
Communication can never be overemphasized. This especially goes for implementations that bring new or changed digital workflows. Many medical devices have IT components that require integrating with existing IT and data solutions such as electronic prehospital patient records, ECG transmissions and more. The data solution must be set up in collaboration with the IT department and in-hospital stakeholders. Here it is key to regularly follow up and report status with a communication plan that the project team approves. Timely communication with key stakeholders and external partners will enhance the likelihood of success.
Phase II: Training, installation, and go-live
When it comes to installation and go-live, hosting workshops in groups and providing manuals are popular methods for educating users. However, some users may need a more flexible learning platform, as they like to be able to do the learning at their own convenience and pace. It is our experience that employing a hybrid learning approach, which offers onsite learning and e-learning (we e.g., have the Medidyne Academy) is effective. We may also complement the in-class and e-learning formats with skill stations, digital manuals, and information shared with the users during the learning journey. The mix of offline and online learning gives the learner an effective learning path. With online elements, the management team can also gain insight with data and dashboards showing completion volume, rate, and more.
Installation of devices should be coordinated with the technical team and end users to ensure the customized settings are ready to use when going live. Plan testing together, supplier and customer, and strive to resolve all critical issues promptly. Still, know that not all go-lives run smoothly, even when you have done the preparation. We e.g., step up our support and availability during go-lives to be ready if unexpected issues surface.
Phase III: Transition to support
The work is not done once the launch is completed. In our experience, continued support and evaluation are important factors for success. When you evaluate how well the device is in use, you can identify potential room for improvement and areas in which more training might be needed. In some instances, you might plan further improvements to the workflow, efficiency, procurement of consumables, etc. Follow-ups can be performed in a variety of ways, e.g., in person and via survey. It is our belief, that having an ongoing dialog with the different stakeholders will bring more positive outcomes for the clinicians and patients.
At Medidyne, we offer end users different kinds of guidance and support after go-live, depending on the medical device implemented: Some devices come with the possibility to download a mobile app with quick guides. For other devices we have video tutorials in local languages and/or regular newsletters with helpful advice. We can also be reached by telephone on urgent support.
Michael Kammer Jensen, Sales Manager at Medidyne, says: “A new medical device is only as effective as the implementation process: If the users are not well-informed about their new product it is often difficult to reap all the benefits that the new, more effective product brings. If users do not experience confidence and trust in using the product after implementation it is unlikely that they will do so when everyday life hits.”
Implementing a new medical device is rarely an isolated event in which a product is simply purchased and put into use by a healthcare professional. Today, IT and connectivity are important features of many devices, and new devices are likely to bring changes to processes and workflows. Therefore, our experience is that applying a holistic approach is effective for achieving success in implementation projects. This includes making a well-thought-out plan with goals, metrics, end user and management buy-in, a solid communication plan, and ongoing evaluation. In all stages of the project, execution becomes more manageable when the right people are involved and engaged.